|

Cancer
is an evolutionary disease fueled by genomic instability. Mammalian
genome integrity is guarded by a complex DNA damage signaling and repair
network, termed the DNA damage response (DDR). ATR is a pivotal kinase
that regulates cell cycle checkpoints and promotes DNA repair processes
via the ATR-Chk1 signaling. In addition to phosphorylation events, other
types of post-translational modifications (PTMs), such as SUMOylation,
ubiquitination, and acetylation are also critical to the ATR-mediated
DDR. Understanding how the ATR signaling and PTM mechanisms are
coordinated to maintain genomic stability will provide insights into
cellular transformation and identify novel therapeutic targets.
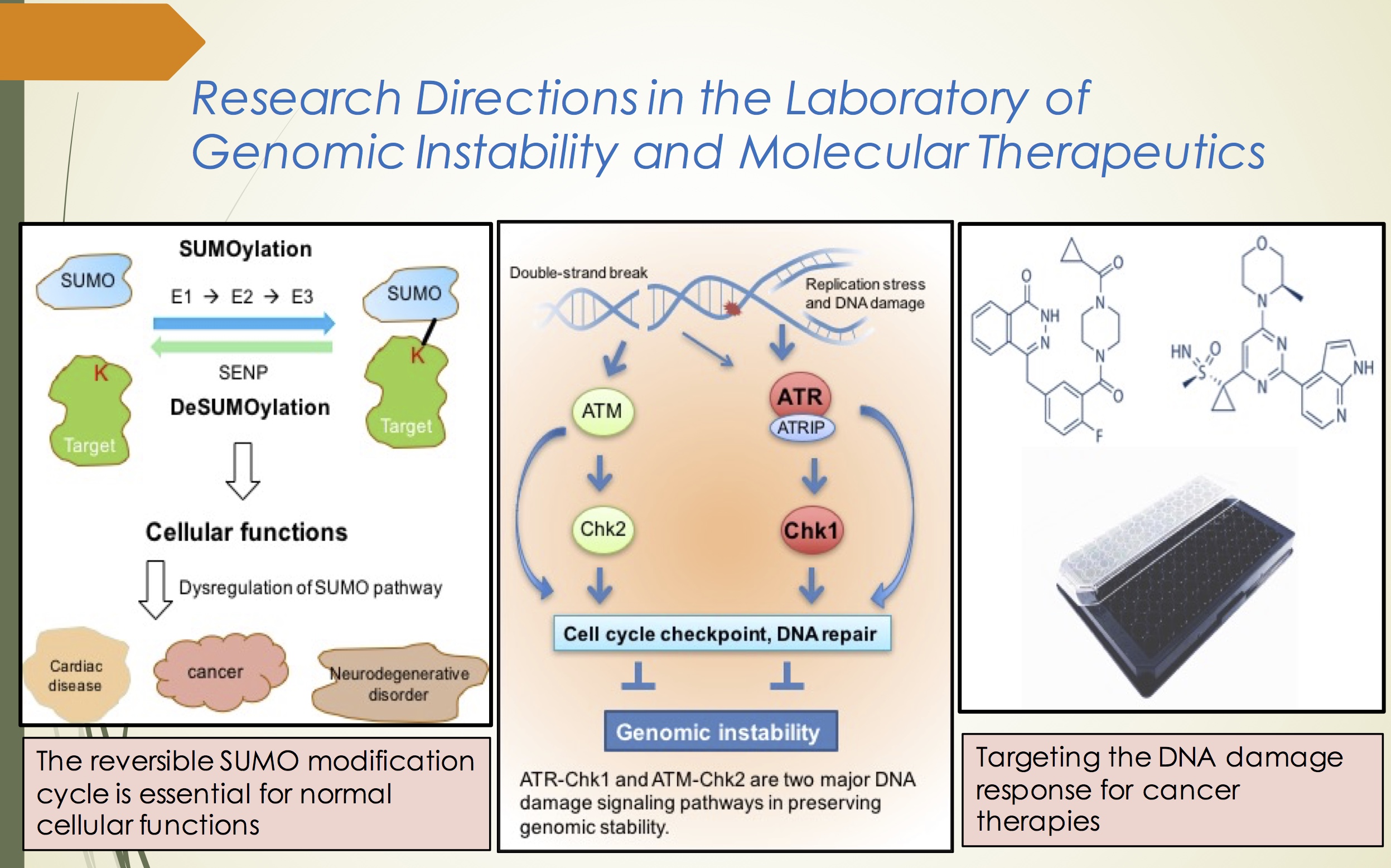
The SUMO
pathway in the ATR-Chk1 signaling
The ATR-Chk1
signaling pathway is essential for regulating the DDR and suppressing
replication stress in the absence of exogenous threats. We showed that
the SUMO pathway directly regulates DNA damage signaling through the
ATR-Chk1 axis. Using approaches of cellular and molecular biology and
protein biochemistry, one of my current research focuses is to dissect
the mechanism by which SUMO pathway regulates DDR and replicative stress
via modulating the ATR-Chk1 signaling.
Developing
novel therapies for treating SUMO-deficient cancers
Inhibition of
the DDR greatly sensitizes cancers to chemo- and radio- therapies, since
most therapies induce DNA damage to kill cancers. The protein level of
SUMO E3 ligases, for example, PIAS3, is commonly reduced or not detected
in certain types of cancers, and those low-PIAS3 cancers are more
resistant to current chemotherapies. We have shown that PIAS3 is
functionally important in ATR signaling and cancer cells with the PAIS3
knockdown by siRNA are more sensitive to inhibitors of PARP and ATR. We
hope to exploit SUMO pathway as prognostic markers and develop novel
therapies using inhibitors of PARP and ATR to target SUMO-deficient
cancers.
近期代表著作:
1]
The SUMO Ligase PIAS3 Primes ATR for Checkpoint Activation. J Bio
Chem 2016,
291:279-90.
[Abstract]
2]
SUMOylation of ATRIP Potentiates the DNA Damage Signaling by Boosting
Multiple Protein Interactions in the ATR Pathway. Genes & Dev
2014, 28:1472-1484.
[Abstract]
3]
PRP19 Transforms into a Sensor of RPA-ssDNA after DNA Damage and Drives
ATR Activation via a Ubiquitin-Mediated Circuitry. Mol Cell
53:1-12.
[Abstract]
This article
is the focus of a research highlight in Nat Rev Mol Cell Biol
2014, 15(2):76.
4]
Targeted Sister Chromatid Cohesion by Sir2. PLOS Genet
2011, 7(2): e1002000. doi:10.1371/journal.pgen.1002000.
[Abstract]
5]
Targeting of Cohesin by Transcriptionally Silent Chromatin. Genes
& Dev 2005, 19, 3031-3042.
[Abstract]
This work is the focus of a dedicated review in Genes & Dev (2006)
20:132-173.
6]
Cordyceps sinensis and its Fractions Stimulated MA-10 Mouse Leydig Tumor
Cell Steroidogenesis. J Androl 2001, 22, 831-837.
[Abstract]
7]
Melatonin Inhibits the Expression of Steroidogenic Acute Regulatory
Protein and Steroidogenesis in MA-10 Cells. J Androl 2001,
22, 245-254.
[Abstract]
|